Andrei K. Yudin

Andrei K. Yudin, Department of Chemistry, University of Toronto, Canada
Short biography: Andrei Yudin is the Tier I Canada Research Chair in Medicine by Design and Professor of Chemistry at the University of Toronto. He received his undergraduate degree from Moscow State University in 1992 and earned his Ph.D. in 1996 at the University of Southern California under the supervision of G. K. S. Prakash and Nobel Laureate George A. Olah. He then pursued postdoctoral studies with Nobel Laureate K. Barry Sharpless at The Scripps Research Institute before beginning his independent career at the University of Toronto in 1998. He was promoted to Associate Professor in 2002 and to Full Professor in 2007. Professor Yudin’s research has pioneered new concepts in chemical reactivity, macrocycle synthesis, and peptide modification, with broad impact on drug discovery and fundamental chemical reactivity. His contributions have been recognized with numerous honors, including the 2025 Liebig Fellowship (University of Giessen, Germany), the 2024 Arthur C. Cope Scholar Award (American Chemical Society), the 2024 IOCF Zen-ichi Yoshida Lectureship (Kyoto University), the 2022 Konrad Adenauer Award from the Humboldt Foundation (Germany), and the 2023 Organic Reactions Lectureship at the University of California–Irvine. In the past 3 years he has also served as a Visiting Professor at Sorbonne University, Hokkaido University, and the Australian National University. He is currently an Associate Editor for Chemical Science (Royal Society of Chemistry).
ISOREACTIVITY IN CHEMISTRY
Andrei K. Yudina
aChemistry Department, University of Toronto
e-mail: andrei.yudin@utoronto.ca
Keywords: isoreactivity, synthetic half-reactions, reaction discovery
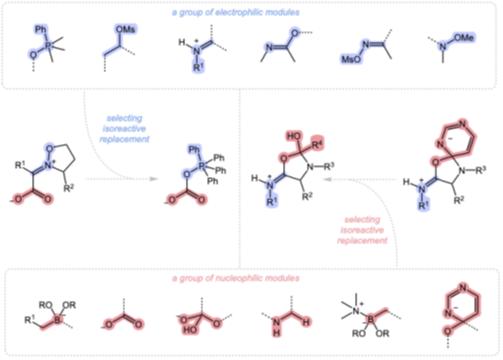
The concept of isoreactivity seeks to reveal parallels in chemical behavior by comparing the functional roles of structural modules within reaction mechanisms. This framework enables systematic analysis of diverse transformations through the replacement of structural modules that preserve reaction viability, even when the underlying mechanism diverges. Isoreactive module replacement is expected to modify intrinsic barriers and thermodynamic contributions to activation profiles while maintaining the overall feasibility of the forward reaction pathway. Isoreactive relationships extend traditional isoelectronic and isolobal analogies by addressing cases where comparable reactivity cannot be adequately captured by existing conventions. The language of functional modules highlights mechanistic relationships that elude the peripheral modifications encoded by typical substituent changes. By illuminating the mechanistic roles of deep-seated, structurally distinct subunits that enable viable reaction trajectories, isoreactivity establishes a practical and inclusive vocabulary for describing chemical reactivity in multicomponent reactions.