Leonid G. Voskressensky

Leonid G. Voskressensky, Patrice Lumumba Peoples' Friendship University of Russia (RUDN University), Moscow, Russia
Title: Electron-deficient alkynes – universal synthons for producing condensed aza-heterocyclic systems
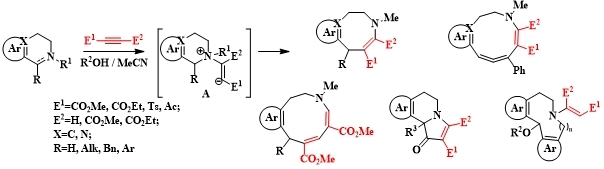
Abstract: In the synthesis of heterocyclic compounds, electron-deficient alkynes hold a prominent position as key reagents. Activated alkynes, due to their ability to undergo a variety of transformations, have found extensive application in reactions involving nitrogen-containing heterocycles. We have developed methods for synthesizing annelated nitrogen-containing rings of medium size, specifically 5- and 6-membered rings, which incorporate pharmacophore groups. These methods utilize electron-deficient alkynes and alkenes and are based on domino and multicomponent reactions. Many of the resulting compounds demonstrate significant bioactivity
Short biography: Prof. Dr. Leonid G. Voskressensky received his PhD degree in Organic Chemistry from the Peoples’ Friendship University of Russia in 1999. In 2001 he joined the Prof. Cosimo Altomare (Universita Degli Studi di Bari, Italy) group as a Post-Doc Fellow (Medicinal Chemistry). In 2001 he became Assistant Professor, in 2006, Associate Professor and in 2011, Full Professor of the Organic Chemistry department of the RUDN. Since 2013, he has been the Dean of the Science Faculty of the RUDN University. The scientific interests of his group mainly focus on heterocyclic chemistry (with special emphasis on indole chemistry), domino reactions methodology, new MCR reactions as well as medicinal chemistry. Prof. Voskressensky has a vast experience in the field of heterocyclic chemistry.
ELECTRON-DEFICIENT ALKYNES – UNIVERSAL SYNTHONS FOR PRODUCING CONDENSED AZA-HETEROCYCLIC SYSTEMS
Voskressensky L.G.
Peoples’ Friendship University of Russia, 117198, Moscow, Miklukho-Maklaya str. 6
e-mail: voskresenskiy-lg@rudn.ru
Keywords:domino reaction, aza-heterocycle, electron-deficient alkynes
In the synthesis of heterocyclic compounds, electron-deficient alkynes hold a prominent position as key reagents. Activated alkynes, due to their ability to undergo a variety of transformations, have found extensive application in reactions involving nitrogen-containing heterocycles.
We have developed methods for synthesizing annelated nitrogen-containing rings of medium size, specifically 5- and 6-membered rings, which incorporate pharmacophore groups [1-4]. These methods utilize electron-deficient alkynes and alkenes and are based on domino and multicomponent reactions. Many of the resulting compounds demonstrate significant bioactivity.
References
[1]. A.V. Listratova, L.G.Voskressensky. Synthesis, 2017, 49, 3801.
[2]. L.G. Voskressensky, T.N. Borisova, A.A. Titov, A.V. Listratova, L.N. Kulikova, A.V. Varlamov, V.N. Khrustalev, G.G. Aleksandrov. Russian Chemical Bulletin., 2012, 61, 1231.
[3]. M. Matveeva, A. Golovanov, T. Borisova, A. Titov, A. Varlamov, A. Shaabani, A. Obydennik, L. Voskressensky. Molecular Catalysis, 2018, 461, 67.
[4]. A.A. Titov, M. Niso, M. De Candia, M.S. Kobzev, A.V. Varlamov, T.N. Borisova, L.G. Voskressensky, N.A. Colabufo, S. Cellamare, L. Pisani, C.D. Altomare. Future Medicinal Chemistry, 2019, 11, 2095.
