Hua Wu

Hua Wu, Shanghai Jiao Tong University, Shanghai, PR China
Short biography: Prof. Dr. Hua Wu is Principal Investigator at the School of Pharmaceutical Sciences, Shanghai Jiao Tong University (SJTU), and a recipient of the National Overseas High-Level Young Talent Program and Shanghai Overseas High-Level Young Talent Program. He earned his PhD from the University of Science and Technology of China in 2014 under the supervision of Prof. Liu-Zhu Gong, then pursued postdoctoral research at Swiss Federal Institute of Technology in Lausanne (EPFL) with Prof. Jieping Zhu, before joining SJTU in May 2021. His research interest centers on novel rearrangement reactions for heterocycle synthesis and structural modification. He has won awards such as the Thieme Chemistry Journal Award and the Chinese Academy of Sciences President's Award.
Rearrangement Reactions-Driven N-Heterocycle Synthesis and Modification
Hua Wu,a Di Tiana and Xing-Zi Lia
aSchool of Pharmaceutical Sciences, Shanghai Jiao Tong University
e-mail: hua.wu@sjtu.edu.cn
Keywords:rearrangement reactions, N-heterocycle synthesis, structural modification, organocatalysis
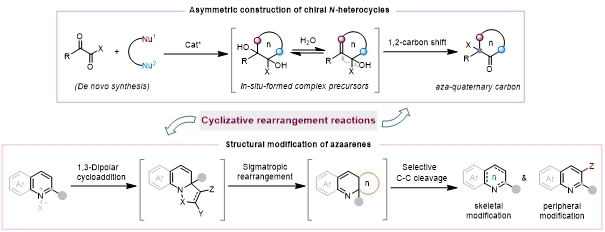
To address the challenges of selective synthesis and precise modification of N-heterocycles, we focus on rearrangement chemistry as the core and propose an "intermolecular cyclizative rearrangement strategy". Through the strategic integration of cyclization and rearrangement events, we
have devised two-component and multi-component reaction systems that in situ generate transient, highly reactive cyclic intermediates. These species then engage in either directed 1,2-rearrangement or concerted rearrangement pathways, thereby enabling both the streamlined synthesis of N-heterocyclic scaffolds and the precise structural modification of azaarenes.
The main research content involves the following two aspects: (1) Based on the cyclizative 1,2-rearrangement strategy of ambielectrophilic-ambinucleophilic reagents, the stereoselective construction of chiral N-heterocycles is realized;[1-5] (2) Based on the 1,3-dipolar cycloaddition/concerted rearrangement strategy, the precise skeleton modification and direct C-H functionalization of azaarenes are achieved.[6-8] This strategy combines the advantages of two reaction types, avoids tedious substrate synthesis, drives interdisciplinary innovation in rearrangement and heterocyclic chemistry.
References
[1]ACIE. 2023, 62, e202217954.
[2] ACIE. 2023, 62, e202313797.
[3] ACIE.2024, 63, e202317182.
[4] ACIE. 2025, 64, e202511791.
[5] JACS. 2024, 146, 26387-26396.
[6] Nat. Chem. 2025, 17, 952-960.
[7] JACS. 2025,147, 30050-30059.
[8] JACS. 2025,147, 41004-41011.
