Zhenghui Kang

Zhenghui Kang, Shanghai Institute of Materia Medica/Zhongshan Institute for Drug Discovery, CAS
Zhenghui Kang, Associate Researcher, Young Principal Investigator, Shanghai Institute of Materia Medica/Zhongshan Institute for Drug Discovery, CAS.
Dr. Zhenghui Kang earned the Ph.D. in Organic Chemistry from East China Normal University in 2018, under the supervision of Professor Wenhao Hu. From July 2018 to January 2022, he conducted postdoctoral research at the School of Pharmaceutical Sciences, Sun Yat-sen University, under the joint guidance of Professor Wenhao Hu. In March 2022, he joined the Shanghai Institute of Materia Medica/Zhongshan Institute for Drug Discovery, Chinese Academy of Sciences, where he serves as an Associate Researcher and Young Principal Investigator. His current research primarily focuses on medicinal chemistry, asymmetric catalysis, and organic synthetic chemistry.
Multicomponent ReactionsEnabled by Metal Carbene Multifunctionalization
Zhenghui Kang,aBaofan Wanga and Wenhao Hub
a Shanghai Institute of Materia Medica/Zhongshan Institute for Drug Discovery,
b Sun Yat-sen University
e-mail:kangzhenghui@simm.ac.cn
Keywords:Metal carbenes, Multicomponent reaction,Interception of two active intermediates
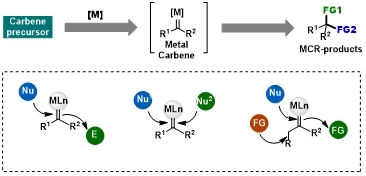
Metal carbenes represent a pivotal class of reactive intermediates in organic synthesis.Capitalizing on the ambiphilic character of the carbene carbon, the direct multifunctionalization of this reactive center enables a versatile platform and a powerful strategy for the development of novel multicomponent reactions, wherein carbene species act as C₁ synthons that couple with two partners via interception of reactive intermediates to afford one-carbon-extended products. Herein, we present three distinct multicomponent reaction (MCR) platforms enabled by the multifunctionalization of metal carbenes. These encompass: (i) sequential electrophilic/nucleophilic carbene carbon functionalization; (ii) transformations initiated by electrophilic capture of the carbene followed by a secondary nucleophilic addition; and (iii) a four-component coupling strategy that achieves triple functionalization of metal carbene intermediates.The applications of this methodology in the synthesis of natural products and pharmaceuticals, as well as the reaction mechanism—particularly the nature of the reactive intermediates and the structure of the metal carbene—will be discussed.
References
[1]For reviews, see: (a) X. Guo, W. Hu. Acc. Chem. Res.2013, 46, 2427–2440; (b) D. Zhang, W. Hu. Chem. Rec. 2017, 17, 739–753.
[2] (a)J. Li, B. Wang, T. Liu, Q. Wen, T. Jing, X. Fu,Y. Pan, K. Wei, X. Zhou, W. Hu, Z. Kang,Nat. Commun. 2025, 16, 6643; (b) Z. Kang, W. Chang, X. Tian, X. Fu, W. Zhao, Y. Liang, X. Xu, W. Hu, J. Am. Chem. Soc.2021, 143, 20818-20827;(c)Z. Kang, Y. Wang, D. Zhang, R. Wu, X. Xu, W. Hu, J. Am. Chem. Soc., 2019. 141, 1473-1478.
[3] J. Li, B. Wang, Q. Wen, X. Zhou, W. Hu, Z. Kang,manuscriptsubmitted.
