Shiliang Shi

Shiliang Shi, Shanghai Institute of Organic Chemistry, PR China
Biography : Shi-Liang Shi received his Ph.D. in organic chemistry from the University of Tokyo in 2011 under the supervision of Prof. Masakatsu Shibasaki and Motomu Kanai. After one year of postdoctoral research with Prof. Motomu Kanai as a JSPS fellow, he joined the group of Prof. Stephen Buchwald at MIT as a postdoctoral fellow. In the summer of 2016, he started his independent research as a professor at the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences. His research interests focus on the design of chiral NHC ligands and their application to challenging enantioselective catalytic transformations from readily available starting materials.
Asymmetric NHC-Metal Catalysis
Shi-Liang Shi
Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
e-mail: shiliangshi@sioc.ac.cn
Keywords:chiral NHCs, asymmetric catalysis, C-H activation, cross-coupling, carbonyl addition
The elaboration of multi-components in a single chemical transformation permits a highly simplified organic synthesis with excellent step-economy and atom-economy; however, controlling the chemo-,regio- and enantio-selectivity in these multicomponent reactions is challenging. Thus, the development of suitable chiral ligands for asymmetric multicomponent transformation is highly demanding. Among all the chiral ligands, N-heterocyclic carbene (NHC) ligands, with their distinctive electronic and steric properties, enable the activation of a range of inert substrates.This holds significant promise for metal-catalyzed synthesis. However, designing chiral NHC ligands with high chiral induction ability is challenging due to their mono-coordination and flexible conformation. The privileged chiral NHC ligand is especially rare, which hampers the advancement of chiral NHC-metal catalysis. This report details the development of novel, induced-fit C2-symmetric chiral NHC ligands, named ANIPE and SIPE, and their application in various challenging enantioselective transition-metal-catalyzed transformations, including alkene functionalization, C-H activation, cross-coupling, and carbonyl addition reactions[1-12].
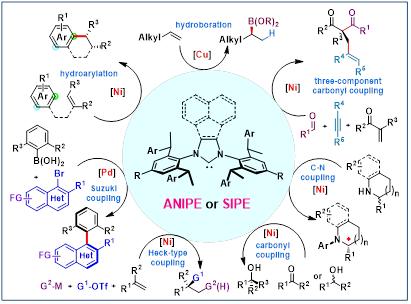
Figure 1. Induced-fit chiral NHC for asymmetric metal catalysis
References
[1] Wang, Z.-C.; Shi, S.-L. Acc. Chem. Res.2025, 58, 2157.
[2] Ruan, L.-X.; Sun, B.; Liu, J.-M.; Shi, S.-L. Science2023,379, 662.
[3] Chen, G.; Liu, J.-M.; Ruan, L.-X.; Shi, S.-L. Nat. Synth.2025, DOI:10.1038/s44160-025-00887-4.
[4] Wang, Z.-C.; Luo, X.; Zhang, J.-W.; Liu, C.-F.; Koh, M. J.; Shi, S.-L. Nat. Catal.2023, 6, 1087.
[5] Sun, B.; Ruan, L.-X.; Shi, S.-L. Nat. Synth.2024, 3, 1091.
[6] Liu, C.-F.; Wang, Z.-C.; Luo, X.; Lu, J.; Shi, S.-L.; Koh, M. J. Nat. Catal.2022, 5, 934.
[7] Wu, H.-Y.; Koh, M. J.; Wang, Z.-C.; Shi, S.-L. Angew. Chem. Int. Ed., 2025, e202503126.
[8] Liu, J.-M.; Ma, X.; Chen, G.; Zhang, D.; Shi, S.-L. Sci. Bull.2025, 70, 674.
[9] Ma, J.-B.; Zhao, X.; Zhang, D.; Shi, S.-L.J. Am. Chem. Soc. 2022, 144, 13643.
[10] Cai, Y.; Shi, S.-L.J. Am. Chem. Soc. 2021, 143, 11963.
[11] Cai, Y.; Ruan, L.-X.; Rahman, A.; Shi, S.-L. Angew. Chem. Int. Ed. 2021, 60, 5262.
[12] Wang, Z.-C.; Xie P.-P.; Hong, X.; Shi, S.-L. Angew. Chem. Int. Ed. 2021, 60, 16077.
