Hanmin Huang

Hanmin Huang, University of Science and Technology of China, PR China
Short biography: Hanmin Huang was born in Hubei, China, and completed his M.S. degree at the Huazhong University of Science & Technology. He obtained his Ph.D. degree in 2003 from the Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS). He then moved to Nagoya University and worked as a JSPS postdoctoral research fellow with Professor Masato Kitamura. In April 2008, he returned to China and initiated his independent research at the Lanzhou Institute of Chemical Physics, CAS as a full professor financed by the ‘‘Hundred-Talent’’ program of CAS. In March 2016, he moved to the University of Science and Technology of China (USTC) as a chair professor. His current research interests are focused on the chemistry of organometallic complexes stabilized by adjacent heteroatoms. Particular attention is being paid to the design of new catalysts and leading complexes as key intermediates for developing novel synthetic methodologies and efficient transformation of nitrogen-containing molecules.
Aminoalkyl Cyclopalladated Complex: Discovery and Synthetic Applications
Hanmin Huang
Department of Chemistry, University of Science and Technology of China
e-mail: hanmin@ustc.edu.cn
Keywords:cyclopalladated complex, aminoalkylation, C-N bond metathesis, adaptive DKR
The rapiddevelopment of transition metal catalysis has drastically expanded chemists’ capability to construct chemical bonds, enabling a broad range of efficient carbon−carbon and carbon−heteroatom coupling protocols that are highly desirable for synthetic organic chemistry. Such remarkable success can be largely attributed to the discovery and identification of new reactive organometallic intermediates and insights into the fundamental chemistry of these well-defined “leading complexes”. In this context, it is recognized that the discoveries, preparations, and detailed reactivity investigations of these well-defined organometallic “leading complexes” are vitally important to the development of new and effective catalytic transformations, and to tackling the challenges in synthetic chemistry in general. In past ten years, we have developed a “nitrogen-containing C-bound complex” as the key intermediate for new catalytic aminoalkylation reactions, in which the ligand moiety was C-bound instead of N-bound to the late transition metal center.[1] The complex could introduce an electron-donating amine group from metal−aminoalkyl species into the target product through C−C bond formation instead of through C-N bond construction via late transition metal-amido species. With these general concepts in mind, our group designed and prepared a Pd−aminoalkyl complex, which has exhibited unique and versatile reactivities in various types of aminomethylation reactions. In this talk, we will present our recent progress in this project.[2]
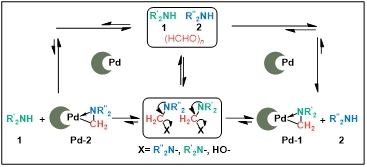
Scheme 1
References
[1]Zhang, H.; Jiang, T.; Zhang, J.; Huang, H. Acc. Chem. Res. 2021, 54, 4305-4318.
[2] For selective examples: (a) Xie, Y.; Hu, J.; Wang, Y.; Xia, C.; Huang, H. J. Am. Chem. Soc.2012, 134, 20613-20616. (b) Xie, Y.; Hu, J.; Xie, P.; Qian, B.; Huang, H. J. Am. Chem. Soc.2013, 135, 18327-18330. (c) Hu, J.; Xie, Y.; Huang, H.Angew. Chem., Int. Ed.2014, 53, 7272-7276. (d) Qin, G.; Li, L.; Li, J.; Huang, H. J. Am. Chem. Soc.2015, 137, 12490-12493. (e) Liu, Y.; Xie, Y.; Wang, H.; Huang, H. J. Am. Chem. Soc.2016, 138, 4314-4317. (f) Yu, B.; Zou, S.; Liu, H.; Huang, H. J. Am. Chem. Soc. 2020, 142, 18341-18345. (g) Chang, R.; Cai, S.; Yang, G.; Yan, X.; Huang, H. J. Am. Chem. Soc. 2021, 143, 12467-12472. (h) Zou, S.; Yu, B.; Huang, H. Chem Catal. 2022, 2, 2034-2048.(i) Zou, S.; Yu, B.; Huang, H. Angew. Chem., Int. Ed.2023, 62, e202215325. (j) Zou, S.; Zhao, Z.; Huang, H. Angew. Chem., Int. Ed.2023, 62, e202311603. (k) Yan, X.; Yu, B.; Liu, H.; Huang, H. Angew. Chem., Int. Ed.2023, 62, e202316563. (l) Cai, S.; Zhao, Z.; Yang, G.; Huang, H. Nat. Chem.2024, 16, 1972-1981; (m) Zou, S.; Zhao, Z.; Yang, G.; Huang, H. Nat. Commun.2024, 10477; (n) Yu, M.; Zhou, Y.; Huang, H.; Angew. Chem., Int. Ed.2025, 64, e202522191; (o) Cai, S.; Zhao, Z.; Yang, G.; Huang, H. J. Am. Chem. Soc.2025, 147, 28624-28631. (p) Yu, B.; Huang, Y.; Zhang, H.; Huang, H. Nat. Chem. 2025, 17, 1256-1264;
