Rongrong Hu

Rongrong Hu, South China University of Technology, PR China
Short biography: Rongrong Hu is a Professor of Stable Key Laboratory of Luminescent Materials and Devices at South China University of Technology. She obtained her B. S. degree at Peking University and her Ph.D degree at Hong Kong University of Science and Technology. She started her independent career at South China University of Technology in 2014, and was promoted to full professor in 2016. She became Fellow of Royal Society of Chemistry in 2021, and served as the associate editor of Polymer Chemistry since 2019. Her research interests are establishing new polymerization reactions, exploring new polymer structures, and developing new polymer materials. Her research group has developed more than 30 types of multicomponent polymerizations, including a series of elemental sulfur-based multicomponent polymerizations, which could directly convert sulfur to a large number of sulfur-containing functional polymer materials efficiently. She has published more than 150 SCI papers, including JACS, Chem, Chem. Sci., Macromolecules, which been cited for more than 10000 times. She has been the recipient of Hanwha-Total IUPAC Young Scientist Award and Young Chemist Award of Chinese Chemistry Society, and is funded as Distinguished Young Scholar from National Science Fund of China.
ELEMENTAL CHALCOGEN-BASED MULTICOMPONENT POLYMERIZATIONS
Rongrong Hua
aState Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou, 510641
e-mail: msrrhu@scut.edu.cn
Keywords: multicomponent polymerization, elemental sulfur, selenium, sulfur-containing functional polymer
Chalcogen-containing polymers have attracted increasing attention, owing to their fascinating properties such as high refractive indices, metal coordination ability, self-healing capability, optoelectronic property, and so on. Currently, the lack of economic monomers and efficient synthetic approaches are the main challenges for the development of chalcogen-containing polymers. Elemental sulfur with large surplus from worldwide petroluem industry, and elemental selenium as byproduct from metal refinery industry, are hence idea sources for the preparation of chalcogen-containing polymers, despite of the challenges of poor solubility of sulfur/selenium in organic solvents and their toxicity to transition metal catalysts.
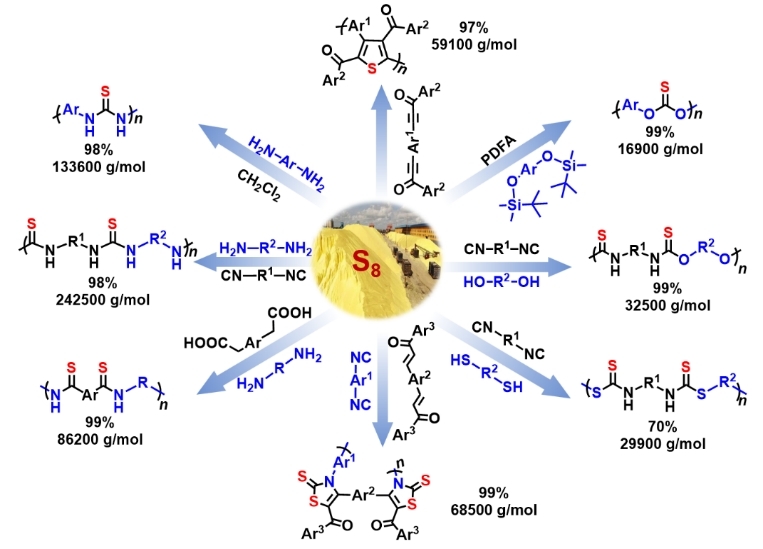
In this talk, a series of elemental sulfur-based multicomponent polymerizations (MCPs) will be introduced to directly convert elemental sulfur to sulfur-containing polymers such as polythioamides, polythioureas, polythiocarbonates, and polythiophenes with well-defined structures, good solubility, high yields, and high molecular weights (Mws) in one step. For example, a KF-assisted MCP of sulfur, CH2Cl2, and aromatic diamines has enabled efficient and economic synthesis of various aromatic polythioureas.[1] Moreover, through the efficient room temperature polymerization of elemental sulfur/selenium and alkynone, non-emissive poly(1,4-dithiin)s/poly(1,4-diselenin)s could be afforded, which could be completely transformed to emissive polythiophenes/polyselenophenes upon heating or oxidation.[2,3] These polymers could exhibit tunable thermal properties, mechanical properties,[4] optical characteristics, and degradability, making them promising candidates for applications including precious metal enrichment and recovery, high-refractive-index materials, and solid-state electrolytes. These MCPs are economic, efficient, and convenient tools for the direct conversion from elemental chalcogen to profitable chalcogen-containing functional polymers, which could accelerate the development of chalcogen-containing polymers with diversified structures and functionalities, demonstrating their great potential in sustainable polymer materials.
References
[1] Y. Huang, Y. Yu, R. Hu, B. Z. Tang, J. Am. Chem. Soc.2024, 146, 14685.
[2] J. Peng, T. Tian, S. Xu, R. Hu, B. Z. Tang, J. Am. Chem. Soc.2023, 145, 28204.
[3] J. Peng, R. Hu,B. Z. Tang,Chem2022, 8, 2301.
[4] L. Chen, R. Hu, B. Z. Tang, J. Am. Chem. Soc.2025, 147, 1134.
