Xiaoming Wang

Xiaoming Wang, Shanghai Institute of Organic Chemistry, Shanghai, PR China
Short biography: Xiaoming Wang received his B.S. in 2009 from Zhejiang University (China). In 2014, he obtained his Ph.D. from Shanghai Institute of Organic Chemistry under the supervision of Prof. Kuiling Ding. In the following years, he did his post-doctoral research with Prof. Frank Glorius at Münster University (Germany) and Prof. Kyoko Nozaki at University of Tokyo (Japan) respectively. Since 2019, he joined Shanghai Institute of Organic Chemistry as a professor. His current research interests focus mainly on the development of novel binuclear metal catalysts and synthetic strategies via bi- and multi-nuclear metallic catalysis.
Biphosphine LigandEnabled Dirhodium-Catalyzed Carbene Difunctionalization
Xiaoming Wang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of ChineseAcademyofSciences, Chinese Academy of Sciences
e-mail: xiaoming@sioc.ac.cn
Keywords:dirhodium,difunctionalization of carbene; three-component reactions, bimetallic catalysis
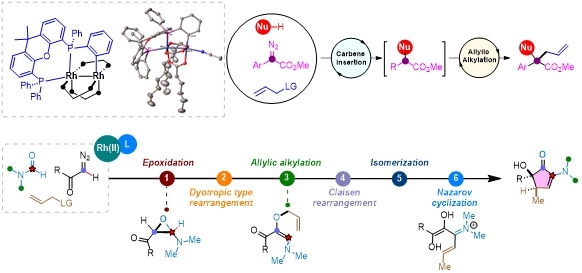
Dirhodium complexes represent a prominent class of dinuclear metal catalysts, widely recognized for their exceptional efficiency in carbene transfer reactions. However, their catalytic versatility has been constrained by the limited ability of dirhodium(II) species to undergo two-electron oxidative addition. To overcome this limitation, we introduced biphosphine ligandsto modulate the electronic and catalytic properties of dirhodium centers. This strategic modification facilitates oxidative addition and expands the reactivity profile of dirhodium beyond classical carbene-mediated transformations.Leveraging this approach, we developed a series of efficient and novel diazo difunctionalization reactions. Notably, a three-component coupling of amines, diazo compounds, and allylic substrates was achieved under dirhodium(II)/Xantphos catalysis, affording α-quaternary α-amino acid derivatives with high atom and step economy. Mechanistic studies support a relay process involving dirhodium-catalyzed carbene insertion followed by allylic alkylation, wherein ligand coordination critically enhances catalytic activity in the allylic alkylation step.Furthermore, with dimethylformamide (DMF) as solvent, the system enabled an unprecedented six-step domino process involving C=O bond cleavage and skeletal reorganization. This transformationcatalyzed by dirhodium/Xantphos, formally inserts a carbenic carbon into the C=O bond of formamide, with concurrent migration of the α-substituent, yielding cyclopentenone derivatives. Key steps include epoxidation, dyotropic type rearrangement, allylic alkylation, Claisen rearrangement, isomerization, and Nazarov cyclization.
References
[1]Lu, B.; Liang, X.; Zhang, J.; Wang, Z.; Peng, Q.*; Wang, X.* J. Am. Chem. Soc.2021, 143, 11799.
[2]Yang, Y.#; Lu, B.#; Xu, G.*; Wang, X.* ACS Cent. Sci.2022, 8, 581.
[3]Wang, G.-Y.; Ge, Z.; Ding, K.; Wang, X.*, Angew. Chem. Int. Ed.2023, e202307973.
[4]Chen, H.; Yang, W.; Zhang, J.; Lu, B.; Wang, X.* J. Am. Chem. Soc.2024, 7, 4727–4740.
[5]Xu, J.; Wang, G.; Ding, K.; Wang, X.* J. Am. Chem. Soc.2025, 147, 2000.
[6] Luo, Y.; Huang, G.; Ding, K.*; Xue, X.-S.*; Wang, X.*Nat. Chem.2025,17,1196.
