Zhenghu Xu

Zhenghu Xu, Shandong University, Jinan, PR China
Short biography:
Zhenghu Xu(徐政虎)
2015.9 – present: Professor, Shandong University
2011.1-2015.9: Associate Professor, Shandong University
2007.10 – 2010.11: Postdoc, Miami University. with prof. Hong Wang
2001.9 – 2007.7: Ph.D. Shanghai Institute of Organic Chemistry
with Prof. Lixin Dai & Yong Tang
1997.9 – 2001.7 B.S. Nanjing University
Research Summary:Tandem Metal Relay Catalysis to polycycles
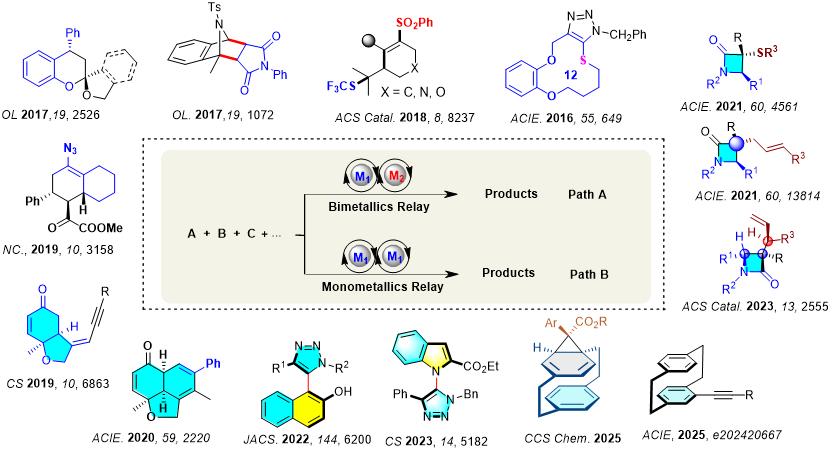
Asymmetric Metal Relay Catalysis to Planar chiral [2.2]paracyclophanes
Zhenghu Xu
aDepartment of Chemistry, Shandong University, Jinan, China
e-mail: xuzh@sdu.edu.cn
Keywords:Asymmetric Catalysis, Relay, Planar chiral, paracyclophane
Planar chirality found tremendous use in many fields, such as chemistry, optics, and materials science. In particular, planar chiral [2.2]paracyclophanes (PCPs) are a type of structurally interesting and practically useful chiral compounds bearing unique electronic and photophysical properties and thus have been widely used in π-stacking polymers, organic luminescent materials, and as a valuable toolbox for developing chiral ligands or organocatalysts. However, the synthesis of chiral PCP derivatives remains a longstanding challenge. Current synthetic methods primarily rely on chiral preparative liquid chromatography separation or chemical and kinetic resolution reactions. Here, we report an enantioconvergent alkynylation of an in situ-formed dehydro-[2,2]-paracyclophane intermediate by asymmetric copper(I) catalysis. This approach enables the efficient synthesis of valuable planar chiral PCP building blocks and heterocycles with good yields and excellent enantioselectivity. The success of this reaction lies in the development of a practical route to access strained dehydro-[2,2]-paracyclophane intermediates, which can also be utilized in various strain-release nucleophilic or cycloaddition reactions to synthesize diverse functionalized PCPs. DFT calculations of this reaction suggest that the enantioselectivity is determined by the aryne complexation with chiral copper(I) acetylide and the subsequent insertion reaction. We anticipate that this new aryne system and its related synthetic applications will provide a new direction in traditional benzyne chemistry. This approach has the potential to serve as a general platform for constructing planar chiral PCPs and could open new avenues for the challenging construction of planar chirality.
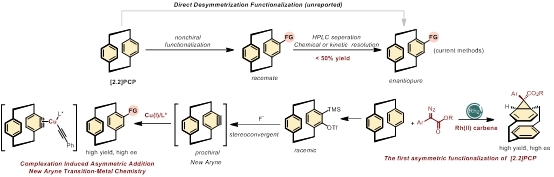
Figure1.Asymmetric Catalytic route to planar chiral [2,2]PCPs
References
[1]D. Chen, Y. Zhou, C.-H. Tung, Z.-X. Yu, Z. Xu, CCS Chem.2025, 7, 1509.
[2] X. Zhang, Y.Zhou, C.-H. Tung, Z.-X. Yu, Z. Xu. Angew. Chem. Int. Ed.2025, e202420667.
