Guangfan Zheng

Guangfan Zheng, Northeast Normal University, Changchun, PR China
Short biography:
Guangfan Zheng was born in 1988 in Yanbian, Jilin, China. He is currently a Professor and Doctoral Supervisor at the School of Chemistry, Northeast Normal University (NENU). He earned his B.S. in Chemistry from Jilin University in 2010, and completed his Ph.D. at NENU in 2017 under the guidance of Professors Qian Zhang and Haizhu Sun. Following his Ph.D., he served as an Assistant Researcher at the Dalian Institute of Chemical Physics, Chinese Academy of Sciences (2017–2018). He then transitioned to Shaanxi Normal University as an Associate Professor (2018–2020), where his research centered on C–H bond activation and asymmetric catalysis. In December 2020, he returned to NENU as a faculty member and was promoted to Professor in 2025. His independent research focuses on radical chemistry and asymmetric catalysis, with an emphasis on developing novel radical cascade reactions and catalytic strategies to enable high-value transformations of readily available substrates such as alkenes, aldehydes, and carboxylic acid derivatives. He has published over 30 SCI-indexed papers as first or corresponding authors in leading journals including Nature Commun., Science Adv., Angew. Chem. Int. Ed. and CCS Chemistry. He also contributed a chapter to a handbook on C–H activation. His academic honors include selection for the Organic Chemistry Frontiers Emerging Investigator Series (2022–2023), the Special Support Grant from the China Postdoctoral Science Foundation (2019), and the Outstanding Doctoral Dissertation Award of Jilin Province (2017). Homepage: https://www.x-mol.com/groups/Zheng_Guangfan.
Multicomponent Radical Reactions Leveraging the Persistent Radical Effect.
Guangfan Zheng,a,* Qian Zhanga
aDepartment of Chemistry, Northeast Normal University, Changchun, China
e-mail: zhenggf265@nenu.edu.cn
Keywords:Radical Cross-Coupling, Photocatalysis, Multicomponent Reactions, Di-functionalization, Persistent RadicalEffect
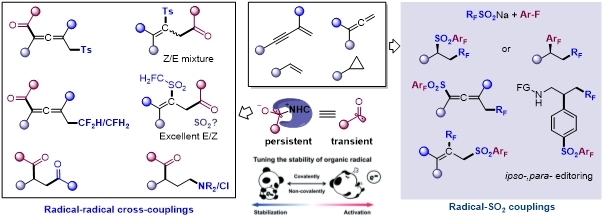
The “persistent radical effect” (PRE) [1] is a kinetic principle that explains the high selectivity often observed in cross-coupling reactions between radical species. This effect arises when two radicals with differing lifetimes are generated at equal rates: the more persistent radical accumulates, while the short-lived species remains at low concentration, leading to preferential cross-coupling.N-Heterocyclic carbenes (NHCs) facilitate the stabilization of acyl radicals, thereby paving the way for controlled radical acylation via radical–radical cross-coupling[2]. Inspired by seminal contributions from Ohmiya, Studer, Chi, and coworkers, we developed a visible-light-mediated dual catalytic system combining NHCs with photoredox catalysts (PCs) to achieve radical acylative difunctionalization of various unsaturated hydrocarbons, including olefins[3b], 1,3-enynes[3c], allenes, and cyclopropanes[3d,e].Furthermore, by employing CF₂SO₂Na as a bifunctional reagent and harnessing the stabilized SOMO of sulfur dioxide, we accomplished controllable difluoromethylation–polyfluoroarylsulfonylation of unsaturated hydrocarbons[3f], as well as ipso-/para-selective difluoromethylation–polyfluoroarylsulfonylation of aniline derivatives[3g].
References
[1]Leifert,D.; Studer,A. Angew. Chem. Int. Ed.2020, 59, 74.
[2]For selected reviews see: (a) Ohmiya, H. ACS Catal.2020, 10, 6862.(b) Liu, K.; Schwenzer, M.; Studer, A. ACS Catal.2022, 12, 11984.(c)Cai, H.; Yang, X.; Ren, S.; Chi, Y. R. ACS Catal., 2024, 14, 8270.
[3](a) Wu, Y.; Sun, J.; Zheng, G.; Zhang, Q. Angew. Chem., Int. Ed.2022, 61, e202117340;(b) Wang, L.; Ma, R.; Sun, J.; Zheng, G.; Zhang, Q. Chem. Sci., 2022, 13, 3169. (c) Wang, L.; Sun, J.; Xia, J.; Li, M.; Zhang, L.; Ma, R.; Zheng, G.; Zhang, Q. Sci. China Chem., 2022, 65, 1938. (d) Li, M.; Wu, Y.; Song, X.; Sun, J., Zhang, Z.; Zheng, G.; Zhang, Q. Nat. Commun.,2024, 15, 8930. (e) Li,M.; Song,X.; Lu,X.; Xia,J.;Zheng, G.; Zhang, Q. Sci. China Chem., 2025, 68, 3628. (f) Xia,J.; Zhu,L.; Lv,Z.; Guo,Y.; Lin,L.; Sun,J.; Zheng,G.; Zhang, Q. Sci. Adv.,2025, Accepted. (g) Teng, B., Guo, Y., Hu L., Zhu L., Jiao K., Xiong T., Zheng G., and Zhang Q., CCS Chem., 2025, Accepted.
